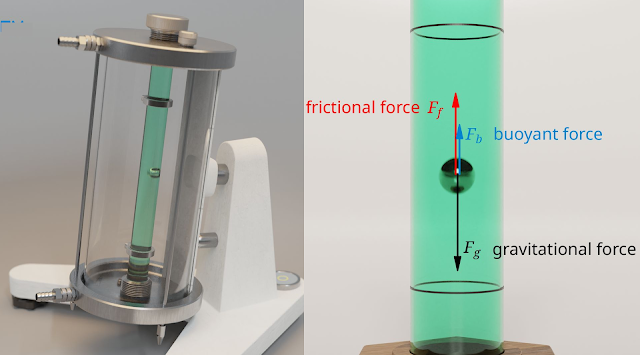
Comparison between Steam Power Plant and Gas Power Plant
In energy generation sector steam power plants are those which make use of a high pressure steam to rotate the turbine and then use that turbine to generate electrical energy through a generator attached to turbine. Whereas the Gas power plant are those which make use of a high pressure gas to rotate the turbine and then use that turbine to generate electrical energy through a generator attached to turbine. The working of the steam turbine power plant is governed and analysed by the Rankine cycle where initially heat energy is used to turn the water inside boiler in to high pressure steam and then that steam is used to rotated the blades of turbine whereas the working of the gas turbine power plant is governed and analysed by the Brayton cycle and directly used the air fuel mixture to burn and produce high pressure gasses which are then used to rotate blades of gas turbine. Steam turbine power plant governed by Rankine cycle starts with the work in put by the pump which provides the sufficient flow rate and pressured head to water. Followed by the boiler that provide all heat input required to generate high pressure and high temperature steam. Steam used by Turbine lower the pressure of the steam which is then condenses to lower the temperature and turning steam into water. Whereas Gas turbine power plant governed by Brayton cycle starts with the work in put by the compressor which provides the sufficient flow rate and pressured head to air. Followed by the burning of air fuel mixture that provides all heat input required to generate high pressure and high temperature gasses. Gasses used by Turbine, lower their pressure which is then exhausted into atmosphere